Overview
| Organism | Homo sapiens, human |
|---|---|
| Tissue | cervix |
| Product Format | frozen |
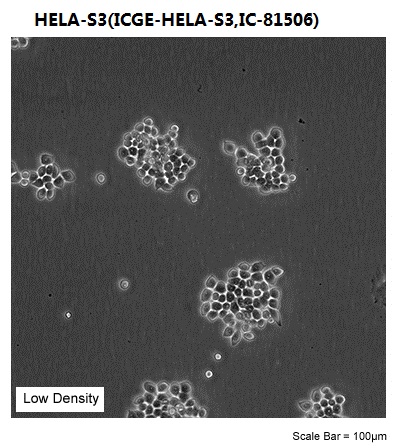
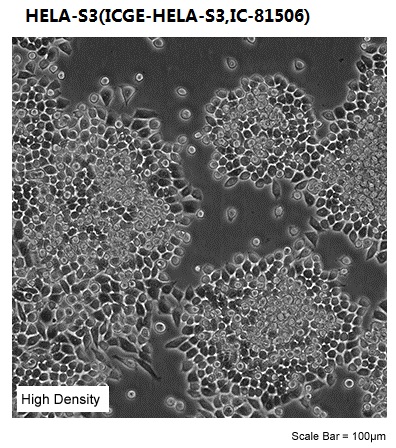
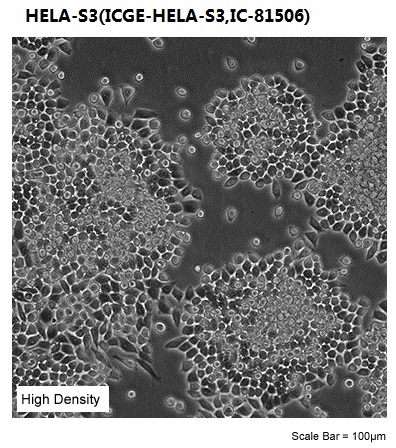
| Morphology | epithelial |
| Culture Properties | adherent |
| Biosafety Level |
2 [Cells contain human papilloma virus (HPV-18)]
Biosafety classification is based on U.S. Public Health Service Guidelines, it is the responsibility of the customer to ensure that their facilities comply with biosafety regulations for their own country. |
| Disease | adenocarcinoma |
| Age | 31 years |
| Gender | female |
| Ethnicity | Black |
| Applications |
This cell line is a suitable transfection host.
The HeLa S3 clone has been very useful in the clonal analysis of mammalian cell populations relating to chromosomal variation, cell nutrition, and plaque-forming ability. |
| Storage Conditions | liquid nitrogen vapor phase |
Properties
| Karyotype | A medium-sized metacentric marker is present in 100% of the cells. HeLa Markers: One copy of M1, one copy of M2, two copies of M3, and one copy of M4. Note: Cytogenetic information is based on initial seed stock at ATCC. Cytogenetic instability has been reported in the literature for some cell lines. |
|---|---|
|
|
|
| Derivation |
HeLa S3 is a clonal derivative of the parent HeLa line (seeATCC CCL-2). S3 was cloned in 1955 by T.T. Puck, P.I. Marcus, and S.J. Cieciura.
|
| Clinical Data |
31 years
Black
female
|
| HeLa Markers | Y |
| Genes Expressed |
The cells are positive for keratin by immunoperoxidase staining.
keratin.
|
| Cellular Products |
keratin
|
| Virus Susceptibility |
Vesicular stomatitis, Glasgow (Indiana) Vesicular stomatitis, Orsay (Indiana) Encephalomyocarditis virus Human adenovirus 5 |
| Virus Resistance |
Human poliovirus 1 Human poliovirus 2 Human poliovirus 3 |
| Comments |
This line can be adapted to grow in suspension.
The cells are positive for keratin by immunoperoxidase staining. A culture at approximately passage 400 was submitted to the American Type Culture Collection in February, 1972. HeLa cells have been reported to contain human papilloma virus 18 (HPV-18) sequences. ATCC confirmed this cell line is positive for the presence of Papillomavirus viral DNA sequences via PCR. |
Background
| Complete Growth Medium |
The base medium for this cell line is ATCC-formulated F-12K Medium, Catalog No. 30-2004. To make the complete growth medium, add the following components to the base medium: fetal bovine serum to a final concentration of 10%. |
|---|---|
| Subculturing |
Volumes used in this protocol are for 75 cm2 flasks; proportionally reduce or increase amount of dissociation medium for culture vessels of other sizes. Flasks do not become 100% confluent. Cells are rounded and have a tendency to float in the medium.
Subcultivation Ratio: A subcultivation ratio of 1:4 to 1:10 is recommended
Medium Renewal: 2 to 3 times per week
|
| Cryopreservation |
Freeze medium: Complete growth medium supplemented with 5% (v/v) DMSO
Storage temperature: liquid nitrogen vapor phase
|
| Culture Conditions |
Atmosphere: air, 95%; carbon dioxide (CO2), 5%
Temperature: 37��C
|


