

| Organism | Homo sapiens, human |
|---|---|
| Tissue | colon; derived from metastatic site: left supraclavicular region |
| Product Format | frozen |
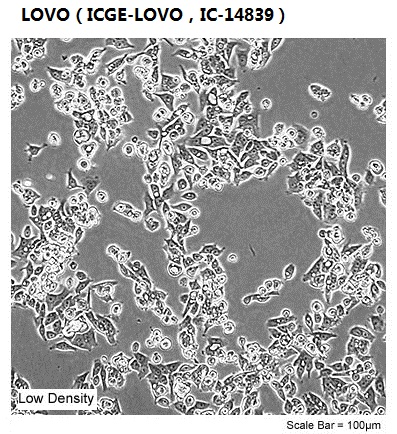
| Morphology | epithelial |
| Culture Properties | adherent |
| Biosafety Level |
1
Biosafety classification is based on U.S. Public Health Service Guidelines, it is the responsibility of the customer to ensure that their facilities comply with biosafety regulations for their own country. |
| Disease | Dukes'''''''' type C, grade IV, colorectal adenocarcinoma |
| Age | 56 years |
| Gender | male |
| Applications |
This cell line is a suitable transfection host.
|
| Storage Conditions | liquid nitrogen vapor phase |
| Karyotype | The stemline chromosome number is hyperdiploid with the 2S component occurring at about 2.7% and 3 marker chromosomes were common to all S metaphases. Karyotypes were generally homogeneous and stable. |
|---|---|
|
|
|
| Derivation |
LoVo was initiated in 1971 from a fragment of a metastatic tumor nodule in the left supraclavicular region of a 56-year-old Caucasian male patient with a histologically proven diagnosis of adenocarcinoma of the colon.
|
| Clinical Data |
male
|
| Antigen Expression | HLA A11, B15, B17, Cw1, Cw3; blood type B |
| Oncogene | myc +; myb + ; ras +; fos +; p53 +; sis -; abl -; ros -; src - |
| Genes Expressed |
carcinoembryonic antigen (CEA) 908 ng/10 6 cells/10 days. The cells are negative for expression of CSAp (CSAp-) and colon antigen 3.
|
| Cellular Products |
carcinoembryonic antigen (CEA) 908 ng/10 exp6 cells/10 days
|
| Tumorigenic | Yes |
| Effects |
Yes, in nude mice
(Tumors developed within 21 days at 100% frequency (5/5) in nude mice inoculated subcutaneously with 10(7) cells)
|
| Comments |
The cells are negative for expression of CSAp (CSAp-) and colon antigen 3.
The line is positive for expression of c-myc, K-ras, H-ras, N-ras, Myb, sis and fos oncogenes.
N-myc and sis oncogene expression were not detected.
Tumor specific nuclear matrix proteins CC-3 and CC-4 are expressed. |
| Complete Growth Medium |
The base medium for this cell line is ATCC-formulated F-12K Medium, Catalog No. 30-2004. To make the complete growth medium, add the following components to the base medium: fetal bovine serum to a final concentration of 10%. |
|---|---|
| Subculturing |
Volumes used in this protocol are for 75 cm2 flask; proportionally reduce or increase amount of dissociation medium for culture vessels of other sizes.
Subcultivation Ratio: A subcultivation ratio of 1:3 to 1:10 is recommended
Medium Renewal: 2 to 3 times per week
|
| Cryopreservation |
culture medium 95%; DMSO, 5%
|
| Culture Conditions |
Temperature: 37��C
Atmosphere: 95% air, 5% carbon dioxide (CO2)
|
| Complete Growth Medium |
The base medium for this cell line is ATCC-formulated F-12K Medium, Catalog No. 30-2004. To make the complete growth medium, add the following components to the base medium: fetal bovine serum to a final concentration of 10%. |
|---|---|
| Subculturing |
Volumes used in this protocol are for 75 cm2 flask; proportionally reduce or increase amount of dissociation medium for culture vessels of other sizes.
Subcultivation Ratio: A subcultivation ratio of 1:3 to 1:10 is recommended
Medium Renewal: 2 to 3 times per week
|
| Cryopreservation |
culture medium 95%; DMSO, 5%
|
| Culture Conditions |
Temperature: 37��C
Atmosphere: 95% air, 5% carbon dioxide (CO2)
|
| Complete Growth Medium |
The base medium for this cell line is ATCC-formulated F-12K Medium, Catalog No. 30-2004. To make the complete growth medium, add the following components to the base medium: fetal bovine serum to a final concentration of 10%. |
|---|---|
| Subculturing |
Volumes used in this protocol are for 75 cm2 flask; proportionally reduce or increase amount of dissociation medium for culture vessels of other sizes.
Subcultivation Ratio: A subcultivation ratio of 1:3 to 1:10 is recommended
Medium Renewal: 2 to 3 times per week
|
| Cryopreservation |
culture medium 95%; DMSO, 5%
|
| Culture Conditions |
Temperature: 37��C
Atmosphere: 95% air, 5% carbon dioxide (CO2)
|


