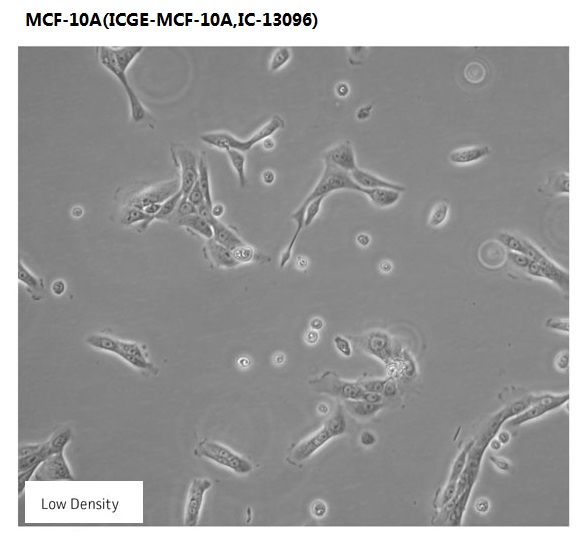
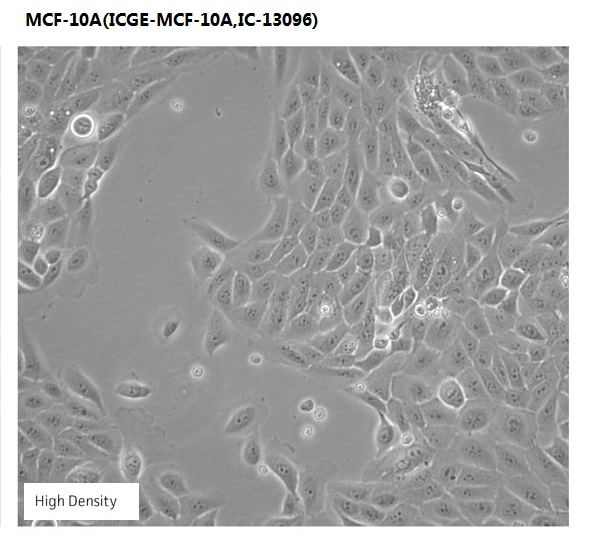
Overview
| Organism | Homo sapiens, human |
|---|---|
| Tissue | mammary gland; breast |
| Cell Type | epithelial |
| Product Format | frozen |
| Morphology | epithelial |
| Culture Properties | adherent |
| Biosafety Level |
1
Biosafety classification is based on U.S. Public Health Service Guidelines, it is the responsibility of the customer to ensure that their facilities comply with biosafety regulations for their own country. |
| Disease | fibrocystic disease |
| Age | 36 years |
| Gender | female |
| Ethnicity | Caucasian |
| Applications |
This line is suitable as a transfection host.
|
| Storage Conditions | liquid nitrogen vapor phase |
| Disclosure | This material is cited in a US or other Patent and may not be used to infringe the claims. Depending on the wishes of the Depositor, ATCC may be required to inform the Patent Depositor of the party to which the material was furnished. This material may not have been produced or characterized by ATCC. |
Properties
|
|
|
|---|---|
| Derivation |
MCF 10A was derived from adherent cells in the population.
|
| Clinical Data |
36 years
Caucasian
female
|
| Tumorigenic | No |
| Effects |
No, in immunosuppressed mice
Yes, in semisolid medium
|
| Comments |
The MCF 10A cell line is a non-tumorigenic epithelial cell line.
The cells are positive for epithelial sialomucins, cytokeratins and milk fat globule antigen.
They exhibit three dimensional growth in collagen, and form domes in confluent cultures.
Thus far, the cells have shown no signs of terminal differentiation or senescence.
The line is responsive to insulin, glucocorticoids, cholera enterotoxin, and epidermal growth factor (EGF).
By electron microscopy the cells display characteristics of luminal ductal cells but not of myoepithelial cells.
They also express breast specific antigens as detected by positive reaction with MFA-Breast and MC-5 monoclonal antibodies.
The calcium content of the medium exerts a strong effect on the morphology of the cells.
|
Background
| Complete Growth Medium |
The base medium for this cell line (MEBM) along with the additives can be obtained from Lonza/Clonetics Corporation as a kit: MEGM, Kit Catalog No. CC-3150. ATCC does not use the GA-1000 (gentamycin-amphotericin B mix) provided with kit. To make the complete growth medium, you will need to add the following components to the kit (sold separately):
|
|---|---|
| Subculturing |
Remove medium and rinse monolayer with PBS (ATCC® 30-2200™). Add 3.0 mL 0.05% trypsin, 0.53 mM EDTA and incubate at 37��C for 15 minutes. To neutralize trypsin, add 3 mL solution of 0.1% soybean trypsin inhibitor (ATCC® 30-2014™). Centrifuge cell suspension at 125 x g for 5 to 10 minutes. Resuspend cell pellet in complete culture medium. Add appropriate aliquots of cell suspension to new culture vessels. Corning® T-75 flasks (catalog #430641) are recommended for subculturing this product.
Subcultivation Ratio: A subcultivation ratio of 1:3 to 1:4 is recommended
Medium Renewal: Every 2 to 3 days
|
| Cryopreservation |
Freeze medium: Complete growth medium supplemented with 7.5% (v/v) DMSO
Storage temperature: liquid nitrogen vapor phase
|
| Culture Conditions |
Temperature: 37��C
Atmosphere: air, 95%; carbon dioxide (CO2), 5% |


